When you really need an economic evaluation in the Medical device development process?
Are you involved in the development of a medical device? Do you wonder when you really need an economic evaluation in the whole development process?
The founders of Betthera published in 2020 an article summarizing the various economics studies needed, in order to enable qualified data-based decisions.
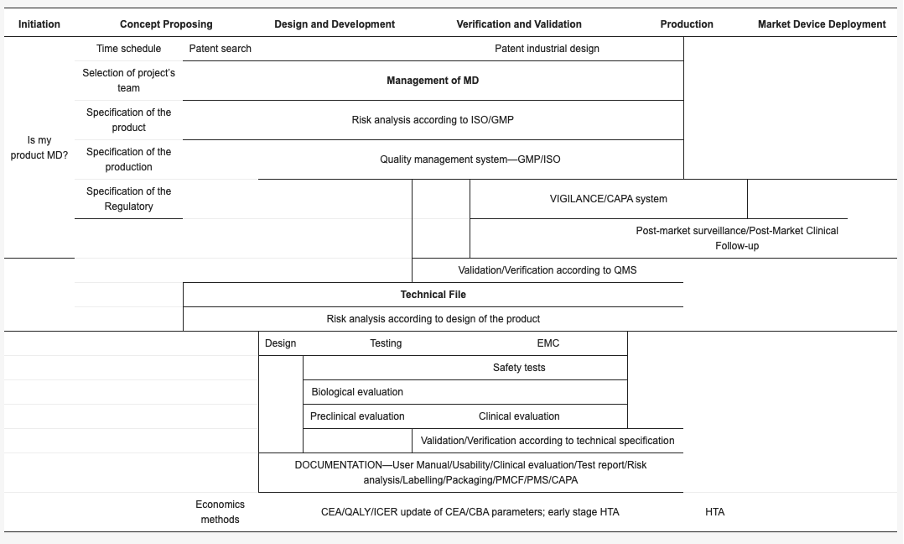
The initiation phase
Comparison of the current situation and the new solution, specification of all benefits and costs, within the general CBA (Cost Benefit Analysis) method, transfer of qualitative benefits to quantitative expression (substitute market methods, shadow prices, hedonic prices, labor market analysis. Depending on the type of medical device (if relevant), a more specific CEA (Cost-Effectiveness Analysis) method may be considered. A cost effectiveness analysis, especially for higher risk classes, should include one QALY (Quality-adjusted life year) calculation, i.e., health if using new intervention/ new technology in place of the original technology. This is a period of preparatory work in which the project is being prepared and decides on its implementation or rejection. In terms of cash flows, this usually includes the costs of project documentation, administrative costs of project preparation, costs of processing economic studies, and costs of evaluating the effectiveness of the investment plan itself.
The content of this phase is in any case the initial calculation of dynamic and static economic indicators as part of the CBA.
In the case of instrumentation (i.e., risk class III), it is advisable to use the HTA (Health Technology Assessment) concept, which includes the assessment of societal impacts, i.e., the CBA method.
The concept proposing, design and development, verification and validation and production phases
These phases require and update of benefits and costs, conversion to financial statements, use of economic indicators: average annual costs, discounted costs, net present value and profitability index, internal rate of return, average profitability (return), payback period.
The medical device market deployment
The final HTA/ CBA calculation with real cost information is necessary. Calculation of sensitivity analysis to identify cash flow scenarios, where its approach may be as follows: characterization of factors, affecting cash flows, change of each factor by a certain percentage, and for each change separately calculation of new indicator value, calculation of change of resulting criterion indicator.
The full article could be found here: Sustainability 2020, 12(5), 1755; https://doi.org/10.3390/su12051755
Do you need your customised plan of economic evaluation for your medical device? Let us know!