SWOT analysis of the EU medical device industry
If we want to build and execute a strategy for any entity, it is often not sufficient to find qualitative inputs, but it has to provide also a prioritisation step. This is also an essential question for policy makers, medical care providers, medical device manufacturers or innovators. The article “The potential of medical device industry in technological and economical context“, published by Betthera founders, show a possibility to quantify the Strengths, Weaknesses, Opportunities and Threats (SWOT) analysis for EU medical device industry.
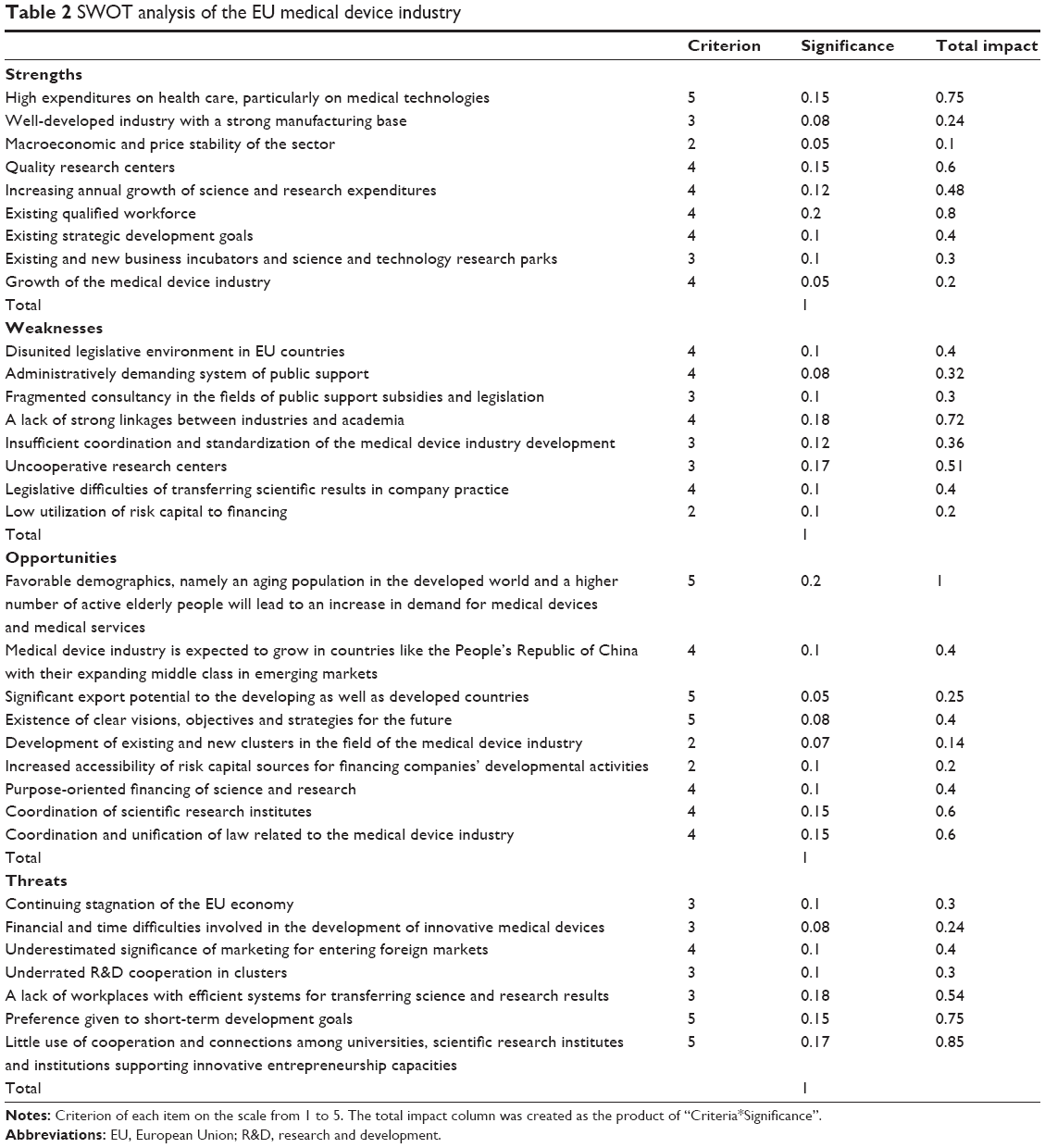
The main findings show that among the most significant strengths of the medical device industry in the EU are, in comparison with other sectors, relatively high expenditures on health care (0.75) and medical technologies, an increasing growth in science and research expenditures, quality research centers, and qualified workforce (0.8).
All factors related to the scattered and dissonant EU administration and legislation (0.4) as well as insufficient interconnection between scientific research institutions and practice (0.72) create obstacles to the growth of medical device industry’s efficiency. In addition, a very crucial weakness is a significant threat to the future when it will be necessary to work on improving the cooperation among universities, scientific research institutes, and institutions, supporting innovative entrepreneurship capacities of science and technology parks. In this industry, it is also essential to pay attention to the long-run development goals. The continuing stagnation of the EU economy (0.1) and underrated R&D cooperation in clusters (0.85) were marked as a threat with relatively smaller significance.
Future opportunities in the medical device industry is aging population (1) and clear vision (0.4) in developed countries. objectives and strategies. Last but not least, also the unification and clarification of legislative requirements.
Do you want to learn more about this SWOT or about our social and business impact assessments, contact us.